MCQ Questions P Block Elements Inorganic Chemistry Chapter 7 Class 12
NCERT Inorganic Chemistry Class – 12 MCQ Practice Questions for Jee Mains | NEET | Class 12
Prepare these Important MCQ Questions of Chapter 7 p-Block Elements in Inorganic Chemistry, Latest questions to expect in Jee Mains | NEET | School Exams.
1. N-N bond is weaker than P-P bond due to repulsion of __
1. Anti bonding electrons
2. Non bonding electrons
3. Bonding electrons
4. None
2. Which among the following has smallest ionic radius?
1. P
2. As
3. N
4. Sb
3. At very high temperature dinitrogen combines with dioxygen to form __
1. NO₂
2. N₂O₅
3. NO
4. N₂O₄
4. Which of the following is ionic fluoride?
1. SbF₃
2. AsF₃
3. BiF₃
4. Both 1 and 3
5. How many group 15 hydrides have enthalpy of formation negative?
a. 2
b. 3
c. 1
d. 0
6. Order of third ionisation of group 15 elements is
1. N > P > As > Bi > Sb
2. N > P > As > Sb > Bi
3. N > As > Sb > Bi > P
4. P > As > Sb > Bi > N
7. The correct order of melting point is:
1. Water > Hydrogen Fluoride > Ammonia
2. Water > Ammonia > Hydrogen Fluoride
3. Hydrogen Fluoride > Water > Ammonia
4. Ammonia > Water > Hydrogen Fluoride
8. The Maximum covalency of oxygen can be __
a. 2
b. 3
c. 4
d. 1
9. Choose the correct statement among the following:
1. All group 16 elements except oxygen show allotropy.
2. All group 15 elements show allotropy.
3. All halogens except Astatine are colored.
4. All group 15 elements are polymorphic in nature.
10. Dinitrogen pentoxide is prepared by treating:
1. Nitric acid with NO.
2. Thermal decomposition of Lead nitrate.
3. Nitric acid with P4O10.
4. All of the above
11. Which of the following acid is used in manufacture of glusose from corn starch?
1. HNO₃
2. HCl
3. H₂SO₄
4. H₃PO₄
12. Select the Correct Statement:
1. Mustard gas have 4 carbon atoms present in its structure.
2. Platinum forms coordination number 4 compound, when dissolved in aqua regia.
3. At high temperature(823K) NaCl reacts with sulphuric acid to form sodium hydrogen sulphate.
4. All of these.
13. Which among the following property of fluorine is less than expected?
1. Ionisation Enthalpy
2. Melting point and Boiling point
3. Melting point and Electronegativity
4. Covalent radius and Electrode potential.
14. S8 reacts with chlorine to form X. Which of the following is correct for X.
1. It has distorted tetrahedron shape.
2. Sulphur has +1 oxidation state in X.
3. X is cyclic structure similar to benzene.
4. There is no S-S bond present in X.
15. Bromine reacts with excess flourine to form compound Y. Y has shape of _
1. Square Planar
2. Bent T
3. Square Pyramidal
4. Pentagonal Bipyramidal
16. Arrange the following in order of their electron gain enthalpy.
1. He > Ne > Ar > Kr > Xe > Rn
2. He > Kr > Xe > Ne > Ar > Rn
3. Ne > Ar > Kr > Xe > He > Rn
4. Ne > Ar > Kr > Xe > Rn > He
17. Choose the correct statements.
1. HCl is used for extracting glue from bones.
2. Oxygen is used in manufacture of many metals, particularly steel.
3. Potassium Permanganate is used in production of ozone.
4. Both 1 and 2.
18. Iodine + excess chlorine reacts to give compound __
1. Orange Solid
2. Black Solid
3. Colorless Gas
4. Ruby Red Solid
19. Which one is most abundant noble gas in atmosphere?
1. Ne
2. He
3. Ar
4. Kr
20. What is color of O₂PtF₆ and XePtF₆ respectively?
1. Red, Blue
2. Both Red
3. Red, Colorless
4. Colorless, Red
21. Choose the odd one out with respect to state of compound (solid, liquid, gas)
1. XeF₂
2. XeO₃
3. XeOF₄
4. XeF₆
22. A sequence of reactions of phosphorous (P₄) is given below, the correct set of products (Q, R, S and T) among the following is
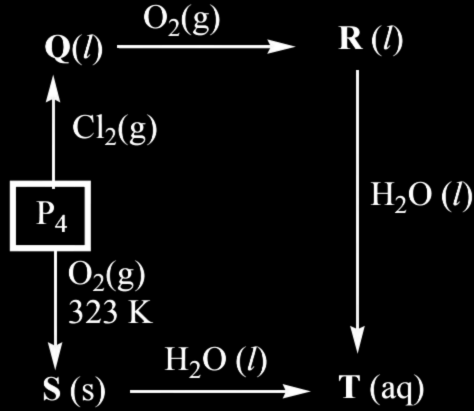
1. Q = PCl₃; R = POCl₃; S = P₂O₃; T = H₃PO₃
2. Q = PCl₅; R = P₂O₅; S = P₄O₆; T = H₃PO₃
3. Q = PCl₃; R = POCl₃; S = P₄O₁₀; T = H₃PO₄
4. Q = PCl₅; R = P₄O₁₀; S = P₄O₁₀; T = H₃PO₄
23. Choose the correct statement.
1. All group 16 elements form hydrides of type H₂E.
2. All hexafluorides of group 16 elements are in liquid state.
3. DIchloride of selenium disproportionate to form compounds of +6 and -2 oxidation state.
4. SeO₂ is found in liquid state.
24. Which of the following metals occurs in nature s decay product of uranium and thorium?
1. Livermorium
2. Polonium
3. Moscovium
4. Oganesson
25. Bond angle in ozone is:
a. 135
b. 120
c. 107
d. 117
26. Cyclo-S6 ring adopts which form
1. Half chair form
2. Twisted form
3. Boat form
4. Chair form
27. Choose the incorrect statement.
1. Bond length in cyclo-S6 form is less than that of S8.
2. Monoclinic sulhphur has higher MP than that of rhombic sulphur.
3. Monoclinic sulhur has lower specific gravity than that of rhombic sulphur.
4. Bond angle is greater in S8 than in cyclo-S6.
28. Chalcogen is greek word for
1. Copper
2. Oxygen
3. Sulphur
4. Brass
29. Which Interhalogen is so unstable that it has been just spectroscopically detected?
1. IF
2. IBr
3. ClF₃
4. BrF₅
30. Identify the nitrogen compounds A, B, C, D, and E.
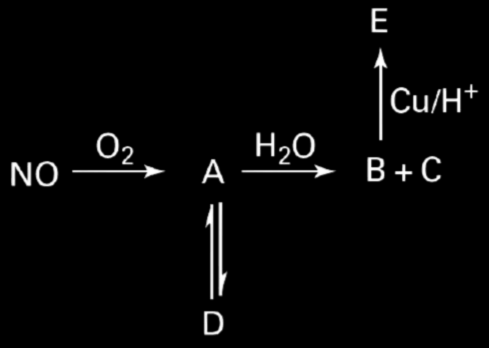
1. A = NO₂ and C = NO₂
2. B = N₂O₄ and D = N₂O₃
3. C = NO and E = NO₂
4. A = NO₂, B = N₂O₃